Publications
Presentations/Lectures
MANUSCRIPT
Transcriptional regulation and cell-specific expression of the mitogen-activated protein kinase-activated protein kinase MK5
Nancy Gerits, Alexey Shiryaev, Sergiy Kostenko, Helle Klenow, Olga Shiryaeva, Mona Johannessen and Ugo Moens*
Department of Microbiology and Virology, Faculty of Medicine, University of Tromsø, N-9037 Tromsø, Norway. *Corresponding author: Ugo Moens, Department of Microbiology and Virology, Faculty of Medicine, University of Tromsø, N-9037 Tromsø, Norway. Phone: +47-77644622; Fax: +47-77645350; E-mail:ugom@fagmed.uit.no
Abstract
The mitogen-activated protein kinase (MAPK) cascades regulate important cellular processes, including growth, differentiation, apoptosis, embryogenesis, motility and gene expression. Although MAPKs mostly appear constitutively expressed, the transcript levels of some genes encoding MAPKs increase upon treatment with specific stimuli. This applies to the MAPK-activated protein kinases MK2 and MK3. In contrast, the transcriptional regulation of the related MK5 has not been studied yet. The MK5 promoters of mouse, rat and human contain a plethora of putative transcription factor sites, and the spatio-temporal expression of MK5 suggests inducible transcription of the gene. We examined the transcription pattern of MK5 in different tissues, and studied the kinetics of MK5 expression at the transcriptional and/or translational level in PC12 cells exposed to arsenite, the cAMP elevating agent (forskolin), KCl, lipopolysaccharide, spermine NONOate, retinoic acid, serum, phorbol ester TPA, temperature shock, and vanadate. Cells exposed to forskolin display a transient increase in MK5 mRNA transcript levels, despite unaltered MK5 protein levels. The MK5 promoters of human, mouse and rat contain a non-consensus cAMP-responsive element that binds the cAMP-responsive element-binding protein (CREB) in vitro. Luciferase reporter constructs containing a 850 base-pair CRE-fragment of the human promoter showed a basal activity 10-fold higher than the constructs in which the CRE-element was deleted. Depletion of CREB by siRNA had no effect on the endogenous MK5 protein levels. Several binding motifs for the heat shock factor are dispersed in the mouse and rat promoter, and temperature shock transiently enhanced the MK5 transcript levels. None of the other stimuli could elicit an effect on MK5 mRNA or protein levels. In conclusion, our results indicate an inducible regulation of MK5 transcription in response to specific stimuli. However, MK5 protein levels remained unaffected for all stimuli tested. The mechanism explaining the discrepancy between increased mRNA levels and unchanged protein levels remains elusive.
Introduction
Protein kinases govern protein phosphorylation, a major post-translational modification that dictates the function of a protein. These kinases catalyze the transfer of the °-phosphoryl group of ATP to hydroxyl groups of specific serine, threonine, and/or tyrosine residues, whereas protein phosphatases, remove the phosphate groups. More than 500 protein kinase-, and more than 150 protein phosphatase-encoding genes, have been identified in the human genome (23).
One major group of protein kinases are the mitogen-activated protein kinases (MAPKs). Initially, the MAPK cascade seemed to exist of a tripartite module in which the most upstream protein kinase, the MAPK kinase kinase (MAPKKK or MEKK), activated a MAPK kinase (MAPKK or MEK), which in turn activated the MAPK. A MAPK then phosphorylated non-protein kinase substrates. However, the discovery of protein kinases that act as substrates for MAPKs blurred the nomenclature of the protein kinases in this tripartite cascade. To avoid renaming the MAPKs, these newly discovered protein kinases were called MAPK-activating protein kinases (MAPKAPKs). Based on the homology of the kinase catalytic domain, the MAPKAPKs can be divided into the subfamilies RSK (ribosomal S6 kinases or MAPKAPK1), MSK (mitogen-and stress-activated kinases), MNK (MAPK-interacting kinases), and the MK (MAPKAPKs). In mammalian cells there exist at least four typical MAPK cascades. The Raf-MEK1/2-ERK1/2 pathway mainly transmits signals from growth factors and cytokines, while stress-inducing signals activate the MEKK1/4-MKK4/7-JNK and the MEKK1/4-MKK3/6-p38 pathways. The MEKK2/3-MEK5-ERK5 pathway can respond to both growth factor- and stress-induced signaling. A fifth atypical pathway consists of the ERK3/4-MK5 module, with its uncharacterized upstream activators and signals (12,32).
Since many mapk-encoding genes display a constitutive expression, the details of mapk promoters and their gene transcription remains poorly understood. Transcript levels of some MAPKs are, however, inducible. The expression of the rice MAPK genes OsBIMK1 and BWMK1 is affected by abiotic and biotic stress (8,40), while the rice MAPKs osMAPK2 and osMAPK4 transcript levels accumulated after cold stress, high salt stress, and under sugar starvation, respectively (9,6). Transcript profiling of tissues derived from untreated and stress-exposed Arabidopsis thalania revealed 2,715 differentially expressed genes, including the MAPKs MKK9 and MAPKKK14 (21). Temperature and salt stress also induced the expression of three other Arabidopsis thalania MAPK genes (25). In Xenopus laevis oocytes, steroid hormone treatment enhanced transcript levels of MOS, the upstream activator of MEK1 (33). Less is known about the transcriptional regulation of mammalian MAPKs. Patients suffering from surgical trauma displayed enhanced ERK5 (=MAPK7) transcript levels, although the exact stimuli and mechanisms for increased transcription of this gene are lacking (38). Microarray studies comparing platelet derived growth factor-treated and untreated NIH3T3 cells and lungs from rats exposed to diesel exhaust particles and control animals revealed elevated transcript levels of MK2, which together with MK3 and MK5 form a subfamily of MAPKAPKs (35,4). Exposure of PC12 pheochromocytoma cells to KCl, forskolin, the calcium ionophore A23187, and ATP, strongly increased MK2 mRNA, while phorbol esters triggered a moderate increase. MK2, as well as the closely related MK3 mRNA levels also augmented in rat ovary after injection of human chorionic gonadotropin (22). Kainic acid also induced MK2 mRNA levels in rat brain while interleukin-6, nerve growth factor or epidermal growth factor did not exert any effect on MK2 transcript levels. None of the stimuli modulated the mRNA levels of MK3 in PC12 cells, while the mRNA levels of the third MK member, MK5, were not examined (42). In this study, we examined the MK5 mRNA and protein levels in different cell lines treated with 10 different stimuli and studied the induction of the 5' flanking region of the mk5 gene. Our results show a tissue-specific transcription pattern of MK5 and suggest an inducible transcription of the gene in response to specific stimuli. However, none of the stimuli tested here affected the MK5 protein levels. The molecular mechanism for the discrepancy between increased mRNA levels and unchanged protein levels, as well as the biological relevance remains unknown.
Materials and methods
Materials
Forskolin, retinoic acid, LPS, sodium arsenite, vanadate and 12-O-tetradecanoyl-13-acetate (TPA) were all purchased from Sigma Aldrich (St. Louis, MO, USA). Spermine NONOate was obtained from Calbiochem, Merck, while KCl was purchased from Merck (Merck Chemicals Ltd, Nottingham, UK). 32P °-dCTP was obtained from Amersham/GE Healthcare (Buckinghamshire, UK). Anti-PRAK (A-7), antibodies were from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA), while anti- CREB (#9104) was purchased from Cell Signaling (Beverly, MA), while anti-GFP (ab290) was purchased from AbCam (Cambridge, UK). The alkalic phosphatase-conjugated secondary antibodies sheep anti-mouse IgG and anti-rabbit IgG were from Sigma Aldrich. Oligonucleotides were purchased from Sigma Aldrich or Eurogentec (Seraing, Belgium). Purified CREB protein was from Active Motive (Carlsbad, CA, USA).
Plasmids
The plasmids pTAL-LUC and pCRE-LUC were from Clontech (Clontech, Takara Bio Inc, Shiga, Japan). The plasmid pCREMK5-LUC was generated as follows: the complementary oligonucleotides (the CRE motif is underlined) 5'-GTA-TGA-CGT-CAT-TAC-TAT-GAC-GTC-ATT-ACT-ATG-ACG-TCA-TTA-3' and 5'-TCG-ATA-ATG-ACG-TCA-TAG-TAA-TGA-CGT-CAT-AGT-AAT-GAC-GTC-ATA-CGT-AC-3' were annealed and ligated into the KpnI/XhoI sites of pTAL-LUC. The transcription of the luciferase reporter gene is driven from a minimal promoter and three copies of the MK5 CRE. The control plasmid pCRE-LUC contains the same promoter but three copies of the consensus CRE motif.
Expression plasmid for VP16 was from Clontech. Expression plasmid for CREB-VP16 was generated by cloning rat CREB cDNA sequences into the EcoRI/BamHI sites of VP16 expression plasmid. For the luciferase plasmid containing the human MK5 promoter fragment, a fragment was amplified from human genomic DNA obtained from whole blood by PCR using the forward primer: 5'-GAC-CTC-CAC-AGA-TCC-TCT-CAG-AAG-GAG-3' (nucleotides 50645-50670 in GI:29126252) combined with the reverse primer starting from the MK5 startcodon: 5'-GCT-TTG-TCC-ATG-TCG-CTC-TCC-TCC-GAC-3'. This yielded a PCR product of 850 bp, which was cloned into TA vector pCR2.1 (Invitrogen). Positive clones were sequenced to verify the presence of the CRE motif. The 850 bp fragment was then subcloned into the KpnI/XhoI sites of the luciferase reporter plasmid pGL3-Basic (Promega). The CRE motif was mutated from TGACGTCA to TCACAAAA using following primers: 5'-GAG-TCC-GGG-CCT-ATC-ACA-AAA-TTA-GCG-CAG-CGC-CAT-3' and 5'-ATG-GCG-CTG-CGC-TAA-TTT-TGT-GAT-AGG-CCC-GGA-CTC-3'. This mutation has been previously shown to prevent CREB binding and to abrogate cAMP response (41). All newly generated plasmids and mutations were confirmed by sequencing. BAC clone was obtained from RZPD German Resource Center for Genome Research (imagines, Berlin, Germany).
Cell culture and transfection
PC12 cells, a kind gift from Dr. Jaakko Saraste (University of Bergen, Norway) were maintained in RPMI-1640, supplemented with 10% horse serum (Gibco) and 5% foetal bovine serum, 2 mM L-glutamine, penicillin (110 units/ml) and streptomycin (100 ¹g/ml). The human neuroblast cell line SK-N-DZ (CRL-2194) was from ATCC (LGC Promochem; Middlesex, United Kingdom) and was maintained in Dulbecco's modified Eagle's medium, supplemented with 10% foetal bovine serum. HEK293 cells were purchased from the European Collection of Cell cultures (cat. no. 85120602; Salisbury, Wiltshire, UK) and kept in Eagle's Minimum Essential Medium supplemented with 10% foetal calf serum, 2 mM L-glutamine, penicillin (110 units/ml) and streptomycin (100¹g/ml). COS-1 cells (ATCC CRL1650) were kept in Dulbecco's modified Eagle's medium, supplemented with 10% foetal bovine serum.
Multi-tissue blot hybridization
The human Multiple Tissue Expression (MTETM) Array was purchased from Clontech (Cat. No. 636901) and the mRNA Array Human Tumor, Array II (192 spots/47 pairs) was from BioChain Institute (Hayward, CA, USA; cat. No.H3235713-1). pcDNA-MK5WT was cut with EcoRI/XhoI to extract the MK5 fragment, after which 50ng of fragment was used for probe labelling with the Random Prime Labelling Module (Amersham) according to the manufacturers instructions. The MTE array was prehybridized in hybridization buffer (5xSSC, 0.1% SDS, 5% dextrane sulphate, 1:20 Liquid block (Amersham)) for 30 min at 45°C. Hybridization of probe and array occurred overnight at 45°C. The next day, the array was washed 4 times in washing solution 1 (1xSSC, 0.05% SDS) at 60°C for 15min, twice for 30 min at 48°C in solution 2 (0.5xSSC, 0.5% SDS) and finally the array was rinsed in diluent buffer (0.1M TrisBase pH9.5, 0.3M NaCl) at RT. Next, the array was incubated for 1h at room temperature in blocking buffer (1:10 dilution of liquid block in diluent buffer), then incubated with anti-fluorescein-AP/BSA for 1h at RT. Subsequently, the array was washed 3 times for 10 minutes in Tween20 washing solution (0.3%Tween in diluent buffer) and rinsed in diluent buffer. For detection, CDP-star was added to the membrane. Detection occurred by exposure to a photosensitive film (Kodak) for 3h.
RNA isolation and reverse transcriptase-polymerase chain reaction (RT-PCR).
Total RNA from transfected HEK293 cells was isolated using the Nucleospin RNA II purification kit (Clontech, Takara Bio Inc, Shiga, Japan) according to the manufacturer's protocol. RNA concentrations and purity were checked by spectrometry on the Nanodrop ND-1000 (NanoDrop Technologies Inc., Wilmington, DE, USA). Two ¹g of RNA was reverse transcribed using iScript cDNA synthesis kit (BioRad, Biocompare, San Francisco, CA) and subjected to PCR. The primers for APRT and the PCR conditions have been previously described (14). The sequences of the hMK5 primers are: 5'-CCC-TAC-ACT-TAC-AAC-AAG-AGC-TGT-G-3' and 5'-CTT-TAT-CTG-TGA-ATC-CAC-GGC-CAT-TC-3'. Thirty cycles of 30 sec at 94°C, 30 sec at 62°C and 30 sec at 72°C were run to amplify hMK5 transcripts.
Electrophoresis mobility shift analysis (EMSA)
EMSA was performed as previously described (26). Briefly, complementary oligonucleotides were annealed in 10xTEN buffer (100mM TrisCl pH7.5, 10mM EDTA, 1M NaCl) and then labelled with 32P °-CTP [3,000 Ci/mmol) with Klenow polymerase. The sequences of the complementary oligonucleotides used in EMSA are (the CRE motifs are underlined):
MK5-CRE: 5'-GTA-CGC-CTA-TGA-CGT-CAT-TAG-CGC-A-3' and 5'-TCG-ATG-CGC-TAA-TGA-CGT-CAT-AGG-3';
c-fos CRE: 5'-CTA-GGA-GCC-CGT-GAC-GTT-TAC-ACT-C-3' and 5'-GAT-CGA-GTG-TAA-ACG-TCA-CGG-GCT-C3';
NF·B: 5'-CGA-GAG-TTG-AGG-GGA-CTT-TCC-CAG-GC-3' and 5'-CGG-AGC-CTG-GGA-AAG-TCC-CCT-CAA-CT-3';
SIE: 5'-CTA-GGA-GCA-GTT-CCC-GTC-AAT-CCC-3' and 5'-GAT-CGG-GAT-TGA-CGG-GAA-CTG-CTC-3';
AP1: 5'-CGC-TTG-ATG-ACT-CAG-CCG-GAA-3' and 5'-TTC-CGG-CTG-AGT-CAT-CAA-GCG-3'; and
MK5-mutCRE: 5'-GTA-CGC-CTA-TGA-CGT-CAT-TAG-CGC-A-3' and 5'-TCG-ATG-CGC-TAA-TGA-CGT-CAT-AGG-3'.
In competitive EMSA, a 100-fold molar excess of unlabelled competitor oligonucleotide was used. Labelled oligonucleotides and CREB protein were mixed in a total volume of 20 ¹l containing 25mM HEPES pH7.5; 12.5mM MgCl2; 100mM KCl; 1mM DTT; 10¹M ZnSO4; 20% glycerol; 0.1% NP-40; 200ng/¹l PolydI-dC (GE Healthcare/Amersham), calf-thymus DNA (0.5¹g/¹l), and 300¹g/ml BSA. Two ng/¹l labelled oligonucleotides and 250ng/¹l recombinant CREB protein (Active Motive) were used. The DNA-protein complexes were allowed to form for 30 min on ice. Then 6x loading buffer (40% glycerol, 50mM EDTA, 0.1% bromophenol blue, 3xTBE) was added to a final concentration of 1x before the samples were loaded on a 5% non-denaturating acrylamide gel. Gels were run for 5 hours at 4°C at 10V/cm in 0.5xTBE. The gel was subsequently dried and subjected to autoradiography.
Transient transfection and luciferase assay
Cells were plated at 3x106 cells per well in a 6-wells plate. Cells were transfected using Lipofectamine 2000 according to the manufacturer's instruction. COS cells were transfected by the DEAE/dextrane method as previously described (24). Luciferase assays were as previously described (14).
Western blotting
For detection of specific proteins, cells were collected in lysis buffer, heated for 10 min at 70°C and sonicated. Samples were then analyzed by SDS-PAGE NuPage 4-12% Bis-Tris SDS-PAGE (Invitrogen) according to the manufacturers protocol and blotted onto a 0.45 ¹m PVDF membrane (Millipore, Billerica, MA, USA). Immunoblotting was performed by first blocking the membrane with PBS-T (PBS with 0.1% Tween-20 (Sigma Aldrich) containing 10% (w/v) dried skimmed milk) for 1 hour and subsequently incubating the membrane with primary antibody. After 4 washes, the membrane was incubated with the appropriate secondary antibody for 1 hour. Visualization of proteins was achieved by using CDP Star (Tropix, Bedford, MA, USA) substrate and Lumi-Imager F1 from Roche (Basel, Switzerland).
Small interfering RNA (siRNA)-mediated depletion of CREB
PC12 cells were plated at 3x106 cell per well into a 6-well dish and validated CREB-directed siRNA or scrambled siRNA (Ambion Inc., Austin, TX, USA), was transfected by Nucleofection (Amaxa) according to the manufacturers instructions using 100 ¹M siRNA/106 cells. Seventy-two hours after transfection, cells were harvest and CREB protein levels were monitored by Western blotting.
Results
MK5 mRNA levels vary in different organs
The original work describing the identification of MK5 revealed that the mk5 gene was transcribed in most of the tissues tested, but the steady-state transcript levels varied in different organs with high expression in brain, heart, liver, skeletal muscle, and kidney and low mRNA levels in the spleen (29,30). MK5 transcripts and protein were also detected in embryos, at 2-, 4-, and 8-cell stages, and in morula and blastocysts during murine preimplantation development (28,31). We extended the investigation of tissue distribution of MK5 transcripts by monitoring MK5 mRNA in multiple adult and foetal tissues by dot blot hybridization. These results are shown in Figure 1 and 2, and our observations together with the findings by Ni et al. and New et al. are summarized in Table 1. The mk5 gene seems to be transcribed in many adult tissues. There were, however, some discrepancies between our results and the previous published results, e.g. we failed to detect MK5 transcripts in liver with both our assays (Clontech and BioChain), which is in contrast with the results of Ni and New and colleagues. Moreover, we did not always obtain the same results with multiple RNA samples of the same tissue on the same multiple tissue arrays. E.g. MK5 mRNA was not always discovered in parallel samples of colon, kidney, lymph node and rectum mRNA that were placed on different areas of the blot (see Table 1, column BioChain). We also investigated foetal brain, heart, kidney, liver, lung, spleen, and thymus and found MK5 transcripts only in foetal kidneys (Figure 1, C11).
The promoters of the mouse, rat and human contain conserved binding motifs for transcription factors
This spatio-temporal distribution pattern of MK5 mRNA suggests specific regulation of the mk5 gene at the transcriptional level. Therefore, we examined the published MK5 promoter region sequences for the presence of consensus binding sites for transcription factors. Computer-aided screening of the ~3300 bp genomic sequence upstream of the ATG start codon revealed several putative transcription factor binding sites for the mouse, rat and human mk5 gene (see Figure 3; (44)). Only the sites that displayed >95% identities with consensus sites are indicated. The presence of such binding sites should be interpreted with care because of the ambiguity of the genomic MK5 sequences deposited in the gene bank and because proteins may bind to non-consensus motifs. All three promoters are GC-rich, with regions containing >90% G or C. This may explain why the reported mouse and rat sequences in the gene bank contain ambiguous sequences. The function and tissue distribution of the transcription factors that may bind the MK5 promoters are summarized in Table 2 and are in accordance with the tissue distribution pattern of MK5. The MK5 promoter in all three species lack a canonical TATA box, while they share two putative cAMP response element (CRE) binding sites. The proximal CRE in the human MK5 (hMK5) promoter has the consensus sequence, while all the others have 1 or 2 mismatches (Figure 3B). The mouse and rat promoter each possess two adjacent putative AP-2 sites. Interestingly, these promoters also contain numerous heat shock factors (HSF) binding sites, while no such sites appear in the human promoter. Because all three promoters share the presence and relative position of the CRE, we first explored the functionality of this element.
CREB binds specifically to the MK5 promoter in vitro
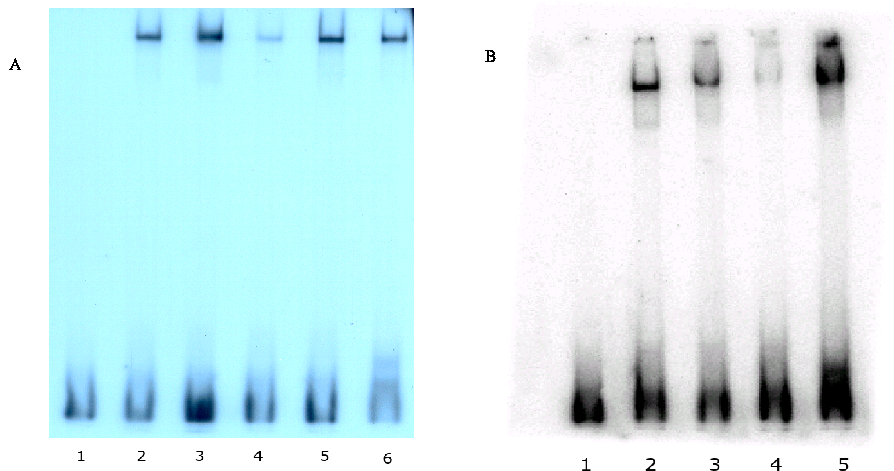
We investigated whether the proximal CRE in the hMK5 promoter could bind CREB in vitro by Electrophoretic Mobility Shift Assay (EMSA). Figure 4 shows that purified CREB protein binds the mouse MK5 CRE motif (lanes 2 in Figure 4A and 4B), as well as to the c-fos CRE motif (lane 6 in Figure 4 and lane 5 in Figure 5B) in vitro. This binding was not disrupted by competing double-stranded oligonucleotides with binding sites for NF·B (Figure 4A, lane 3) serum response factor (Figure 4A, lane 5), or a mutated MK5 CRE motif (lane 3, Figure 4B), while an oligonucleotide with AP-1 binding site strongly interfered with CREB-MK5 CRE binding (Figure 4A, lane 4). This is not unexpected, as the AP-1 protein can bind CRE motifs as well (36).
MK5 transcript levels are transiently increased in response to the cAMP elevating agent forskolin
CREB bound to its cognate CRE motif mediates transcription upon phosphorylation at Ser-133. An event catalyzed by the major CREB kinase, protein kinase A or cAMP-dependent protein kinase (PKA). Forskolin, an activator of adenylate cyclase, increases the intracellular concentrations of cAMP, which induces PKA activation [reviewed in 16. We compared the MK5 mRNA levels in untreated and forskolin treated cells over time and observed a time-dependent increase in MK5 transcript levels in both SK-N-DZ and PC12 cells (Figures 5A and 5B). However, the cell lines differed in the kinetics of the augmentation in MK5 transcripts. In PC12 cells, MK5 mRNA levels peaked 30 minutes after forskolin treatment, remained elevated for 60 min and then returned to basal levels. In SK-N-DZ cells, an increase in transcript levels occurred already after 15 min of treatment and gradually continued until the highest levels were reached 3h after forskolin stimulation. Six hours afterwards, the mRNA levels had returned to basic levels. The transcript levels of the cAMP response genes c-fos, Nur77, and leukemia inhibitory factor (5) were transiently upregulated after forskolin treatment (data not shown).
These increases in MK5 mRNA could result from enhanced transcription from the MK5 promoter by activated CREB. Alternatively or in addition, cAMP may stabilize MK5 transcripts, as has been shown for other mRNA species [reviewed in (15). To distinguish between the two mechanisms, we monitored the forskolin-induced MK5 mRNA levels in the presence of actinomycin D, an inhibitor of transcription. Thereto, we exposed PC12 cells to actinomycin D for 30 min or left them untreated. Then, forskolin was added to all cells and MK5 mRNA levels were determined at different time points. The levels of forskolin-induced MK5 mRNA reduced in the presence of actinomycin D (Figure 6), suggesting that the forskolin-induced increase in MK5 transcripts resulted from enhanced transcription rather than cAMP-mediated stabilization.
The isolated MK5 CRE motif does not mediate cAMP response
Because the proximal hMK5 CRE bound CREB in vitro, we examined whether the isolated CRE motif of the MK5 promoter, when fused to a minimal promoter, could respond to cAMP. Three copies of the MK5 CRE motif were cloned in the pTAL-LUC reporter plasmid to generate the plasmid pCREMK5-LUC. The TAL-LUC plasmid contains a minimal promoter consisting of a TATA-box element. A corresponding plasmid containing three copies of the consensus CRE motif TGACGTCA (pCRE-LUC) was used as a control. Cells were transfected with either of these constructs and treated with forskolin. Our results showed that forskolin treatment induced a >5-fold increase in luciferase activity in cells transfected with pCRE-LUC, while no increase in luciferase activity was observed in pCREMK5-LUC transfected cells. Co-transfection with an expression plasmid for the catalytic C® subunit of PKA tagged with a nuclear localization signal provoked a very potent increase (>100-fold) in luciferase activity in cells transfected with pCRE-LUC, while only a +/-5-fold increase was measured in the cells transfected with pCREMK5-LUC. Expression of a kinase inactive C® mutant (L72H) had only minor (+/-2.5-fold) effects on the strength of both promoters, indicating that an active C® is required to enhance transcription from the consensus CRE-containing promoter (Figure 7).
Phosphorylation of CREB at Ser-133 by PKA is necessary, but not sufficient to activate CREB and stimulate a cAMP-responsive promoter (15,34). It is plausible that the inability of PKA to enhance the transcriptional strength of the promoter in the pCREMK5-LUC reporter plasmid results from the lack of PKA to activate CREB. To test this, we co-transfected cells with the CRE-driven luciferase plasmids (either pCRE-LUC or pCREMK5-LUC) and an expression plasmid encoding CREB-VP16 fusion protein. This protein binds to CRE through its CREB moiety, while the transactivation domain of VP16 augments the transcriptional activity of the promoter independently of CREB. As Figure 8 depicts, we observed a moderate, but reproducible stimulation of transcription by CREB-VP16, driven by a minimal promoter containing 3 copies of consensus CRE motifs (pCRE-LUC; 2-fold), as well as by the promoter with three copies of the MK5 proximal CRE binding sites (pCREMK5-LUC; 1.6-fold).
The proximal consensus CRE motif in the hMK5 promoter is necessary for basal promoter activity, but cannot mediate PKA-induced transcription
The functionality of a CRE may be affected by adjacent sequences or binding motifs for other transcription factors [reviewed in (15). This prompted us to examine the functionality of the CRE motif in the context of the MK5 promoter. Because of the ambiguity of the genomic sequences in gene bank and possibly also because of the very high GC content of the promoter, we could only clone an 850 bp fragment of the hMK5 promoter encompassing the proximal consensus CRE (see Figure 3A and 3B). Transient transfection studies with this promoter fragment linked to the luciferase reporter gene were performed in different cell lines, including PC12, SK-N-DZ, COS-1, and A549 cells. The cloned fragment could induce transcription of the luciferase gene, but remained unresponsive to forskolin in all cell lines tested. Mutation of the putative CRE motif in this MK5 promoter fragment consistently reduced luciferase activity 5- to 10-fold compared to the luciferase activity measured in extracts of cells transfected with the wild-type MK5 promoter fragment (Figure 9A, 9B, 9C and results not shown). This may indicate that CREB is involved in basal MK5 promoter activity. To test this, we monitored MK5 transcript levels in PC12 cells in which CREB expression had been inhibited by treating PC12 cells with siRNA directed against CREB mRNA. Western blot analysis showed a very strong reduction in the amount of CREB protein in cells transfected with siRNA directed against CREB mRNA, but not in cells treated with scrambled siRNA. However, similar MK5 protein levels were observed in both cells (Figure 9D). The results for the effect of CREB siRNA treatment on MK5 mRNA levels are underway (Figure 9E).
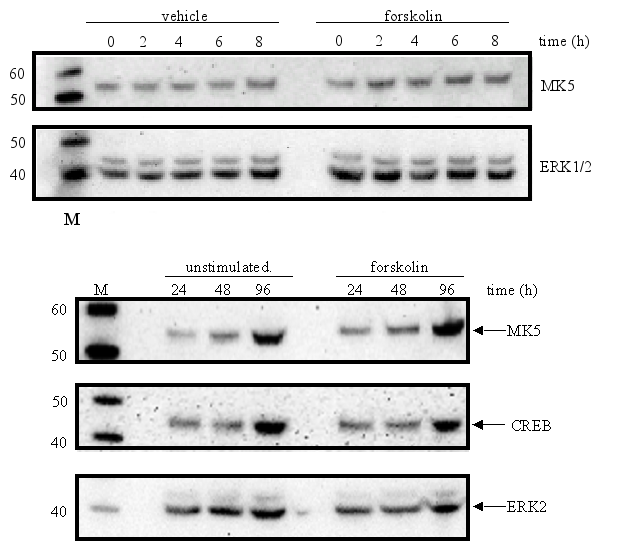
MK5 protein levels are not increased in FSK-treated cells
Next, we examined the levels of MK5 protein in forskolin-treated cells. No increase in MK5 levels compared to untreated control cells was observed in forskolin-exposed cells after 2, 4, 6, 8, 24, 48, and 96 h (Figure 10). The increase in MK5, CREB, and ERK2 levels in vehicle- and forskolin-treated cells at 48 and 96 h was probably due to the increase in the number of cells.
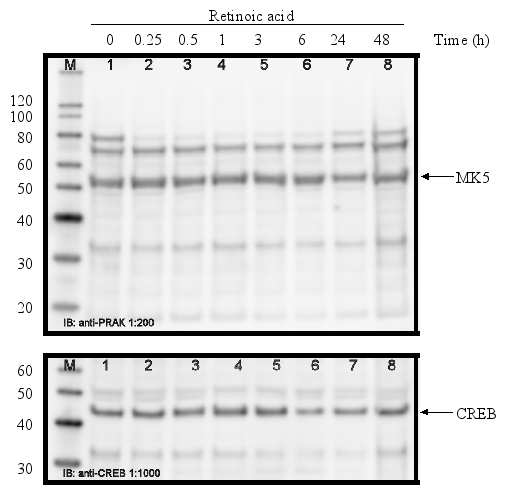
The protein levels of MK5 in PC12 cells remain unchanged after exposure to growth factors, stress, phorbol ester, lipopolysaccharides, and retinoic acid.
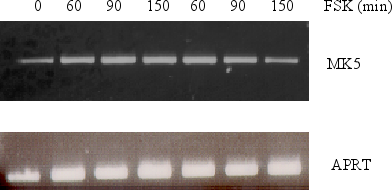
The promoters of the mk5 gene of rat, mouse, and humans contain binding sites for transcription factors that are targets for several different pathways, including the MKK6/p38 MAPK signaling cascade. This stress responsive pathway has been the only pathway until now that can induce MK5 activity in vitro and in vivo (29). This prompted us to study the effect of stress stimuli (such as arsenite, spermineNONO, LPS, vanadate and KCl) that can activate the p38 MAPK pathway, on the expression levels of MK5. In addition, stimuli that target transcription factors with putative binding sites in the MK5 promoters were examined. These included the phorbol ester TPA, which can activate the transcription factors AP1 and AP2 (10,19), and retinoic acid (RA), which regulates the expression of AP2, activates CREB in human tracheobronchial epithelial cells and reduces ERK1 expression levels in squamous cell carcinoma (17,20,2). Moreover, growth factors present in serum may modulate the activity of transcription factors like AP-1, CREB, GATA-1, USF (14,3,11,18). Because of the peculiar characteristic of the multiple HSF bindings sites in the mouse and rat MK5 promoter, MK5 protein levels were also examined in cells exposed to a 44°C temperature shock. As shown in Figure 11A-E, none of the stimuli tested changed the MK5 protein levels compared to untreated cells. It should be noticed that cells treated with vanadate detached shortly after treatment. Because of the discrepancy in MK5 transcript levels and protein levels after forskolin stimulation, we assayed the amounts of MK5 mRNA in untreated cells and cells treated with 60 mM KCl, temperature shock (20 min at 44°C), or 16 nM TPA. No major differences in the MK5 mRNA levels were observed between KCl- or TPA-treated cells and control cells. A decrease in MK5 transcripts was observed after prolonged KCl treatment (1h or longer), but the levels of the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GADPH) gene also diminished at these time points. The reduction was probably the result of reduced viability of the treated cells. MK5 transcript levels increased 3hrs after cells that had been exposed to a 20 min temperature shock of 44°C were replaced to 37°C. The elevated levels remained for at least another 3hrs.
Discussion
The protein levels of most MAPKs seem unaffected by extracellular stimuli, but these signals can provoke activation of MAPKs through post-translational phosphorylation (32). Another mechanism that can control the availability of active MAPKs includes protein stability. For example, the MAPK ERK3 appears constitutively phosphorylated and active but is very unstable, as it becomes almost undetectable in all cells examined. During differentiation, ERK3 protein accumulates, because of increased stabilization (possibly by MK5), which enables it to exert its functions. The signals and mechanism responsible for enhanced ERK3 expression during differentiation remain elusive (39,1). Finally, cell type-specific and stimulus-induced transcription can also control the availability of certain MAPKs (21,25,22,8,9,38,42,33,40,4,6,35). Here, we report that MK5 expression occurs in a spatio-temporal way. However, we observed some discrepancy in the expression pattern of MK5 mRNA in the examined tissues reported by other studies and ours. The quality of the RNA, the species investigated, and the detection method may explain these differences. To unequivocally resolve the expression pattern of MK5, other detection methods such as western blotting, in situ hybridization, immunohistochemistry and reverse transcriptase-PCR should be used. On the other hand, MK5 its spatio-temporal expression pattern and the presence of multiple putative binding sites for cell-specific transcription factors may also account for this.
Since the MK5 promoter contains a CRE motif, CREB may mediate forskolin-induced stimulation of MK5 transcription. Despite clear increases in MK5 transcript levels in forskolin-treated cells, we could not observe augmented MK5 protein levels. The reason for this observation remains unknown, but may be attributed to a lack of nuclear export of MK5 mRNA or prevention of translation of cytoplasmic MK5 mRNA. In immature Xenopus laevis oocytes, the mRNA of the MAPK MOS is not translated into protein until testosterone is added. This hormone induces the interaction of the proteins EG2 and cytoplasmic polyadenylation element-binding protein with the 3'-untranslated region of MOS mRNA which leads to increased polyadenylated levels of MOS mRNA and a subsequent augmentation in MOS protein levels [33 and references therein]. Whether a similar polyadenylation-dependent mechanism operates during FSK-induced elevation of MK5 transcripts, remains unknown since we have not examined the 3'-region of MK5 transcripts in untreated and forskolin-treated cells.
A miRNA-mediated mechanism may also explain the discrepancy between enhanced MK5 transcript levels and unchanged protein levels after forskolin treatment. Indeed, a CREB-induced miRNA, miRNA132, was recently isolated from PC12 cells. Transcription of the miR132 gene occurred rapidly after neurotrophin-induced CREB activation and persisted for at least 24h. There exist several predicted targets for this miRNA, one of those being the GTPase-activating protein p250GAP (43). The involvement of such a mechanism may explain our results as well: forskolin induces the transcription of the mk5 gene and a miRNA (e.g. miR132 or another CREB-inducible miRNA). This miRNA will prevent translation of the MK5 mRNA so that no obvious change in total MK5 protein levels is detected between untreated and forskolin treated cells. It is not known whether MK5 may be a putative target of miR132, but stimulation with forskolin for up to 48h did not induce the MK5 protein levels in PC12 cells. This corresponds well with the long-lasting expression of miR132 in PC12 cells after CREB activation.
In order to find out whether MK5 transcripts can leave the nucleus, we could monitor the subcellular movements of its mRNA. One method to visualize the movement of mRNAs within the cell, relies on the binding of MS2-GFP fusion protein to a specific transcript. This fusion protein consists of the RNA-binding protein of bacteriophage MS2 and green fluorescent protein. To accomplish this, one can fuse several MS2 binding motifs to the 3' untranslated region of an mRNA of interest, which is cloned into a reporter plasmid (7). Thus by transfection cells with an expression plasmid for MS2-GFP and a plasmid containing MK5 mRNA with MS2 binding motifs, the movements of MK5 mRNA can be compared untreated and stimulus-treated cells. Another method that allows detection of transcripts of a specific endogenous gene uses a pair of molecular beacons, one with a donor and the other with an acceptor fluorophore that hybridize to adjacent regions on the same mRNA target, resulting in fluorescence resonance energy transfer (FRET). Detection of the FRET signal allows the localization of specific endogenous RNAs in living cells (37).
Our studies showed that CREB interacted with the MK5 CRE motif in vitro. Chromatin immunoprecipitation studies with PC12, HEK293T and HepG2 cells revealed that CREB is bound to the MK5 promoter in vivo (45,13). However, forskolin treatment in HEK293T cells did not seem to induce the MK5 transcript levels (45). A lack of cAMP response of the MK5 promoter was also found with our transient transfection studies with a reporter plasmid containing an 850 bp fragment of the hMK5 promoter in PC12, SK-N-DZ, COS-1, and A549 cells. One possible explanation could be that the 850 bp MK5 promoter fragment lacked additional elements, that lie upstream in the MK5 promoter or in distant regions, which are required for forskolin-induced gene transcription. For example, the hMK5 promoter possesses an additional distal CRE motif and an AP2 site. The latter can intercede cAMP-induced transcription (10). Three copies of the proximal hMK5 CRE (-758/-751, see Figure 3B) were also unable to make a heterologous promoter cAMP responsive, while three consensus CRE motifs rendered the same promoter cAMP-responsive (e.g. Figure 9A and 9B). A possible explanation may be that the sequences adjacent the hMK5 CRE affect the functionality of the CRE, as has been shown for other CRE motifs ().
A previous study in PC12 cells revealed that MK2 mRNA levels were induced approximately 10-fold after KCl treatment, while forskolin triggered a 20-fold increase in MK2 mRNA (42). The kinetics of forskolin-induction was, however, different from MK5. While MK2 levels increased only 1.5 h after stimulation, peaked after 4h, and remained elevated for at least 24 h, we found that MK5 mRNA levels were highest after 30 min, and returned to control levels after 3 h. This difference may be explained by the distinct regulation of the genes and/or by the use of unlike concentrations of forskolin. We used 10 ¹M forskolin, which is sufficient to provoke maximum PKA activation (27), while Vician et al. used 50 ¹M.
Finally, the MK5 promoters of the different species contain numerous other regulatory elements (Figure 3 and Table 2). This made us test relevant stimuli such as heat shock, TPA, arsenite, spermineNONO, LPS, vanadate, KCl, arsenite, RA, LPS, and serum. None of the stimuli tested had an effect on the MK5 protein levels or RNA levels, except heat shock which increased the MK5 mRNA levels. The presence of multiple HSF binding sites in the rat is in accordance with heat shock-inducible transcription of this gene. HSF sites are lacking in the human promoter, so it would be interesting to test the effect of this stimulus on transcription of the human mk5 gene.
In conclusion, our studies failed to detect stimulus-induced regulation of MK5 expression at the protein level, despite a plethora of transcription factor binding sites in the promoter and a cell- and developmental specific expression pattern of the gene. The regulation of MK5 remains unsolved in many aspects because physiological stimuli that activate this protein kinase remain to be identified. Recently, we have shown that activation of the PKA signaling pathway stimulated phosphorylation and kinase activity of MK5, and provoked nuclear export of MK5. Whether other signal pathways control these activities is not known.
Acknowledgements
This work was supported by grants from the Norwegian Research Council (NFR, project S5228), The Norwegian Cancer Society (Kreftforeningen, project A01037), the Erna and Olav Aakres Foundation, and the Family Blix Foundation.
Figures and tables
Figure 1 : Tissue-specific expression of MK5
A multi-tissue blot with RNA isolated from different tissues was examined for the presence of MK5 transcripts. The top panel shows the results of the hybridization of an MK5-specific probe, while the bottom panel indicates from which particular tissues the RNA on the blot was derived. RNA was isolated from normal adult and embryonic tissues.
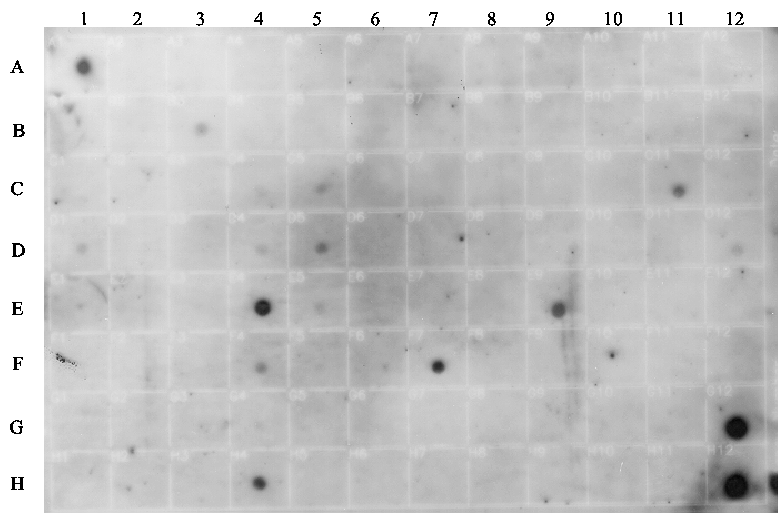
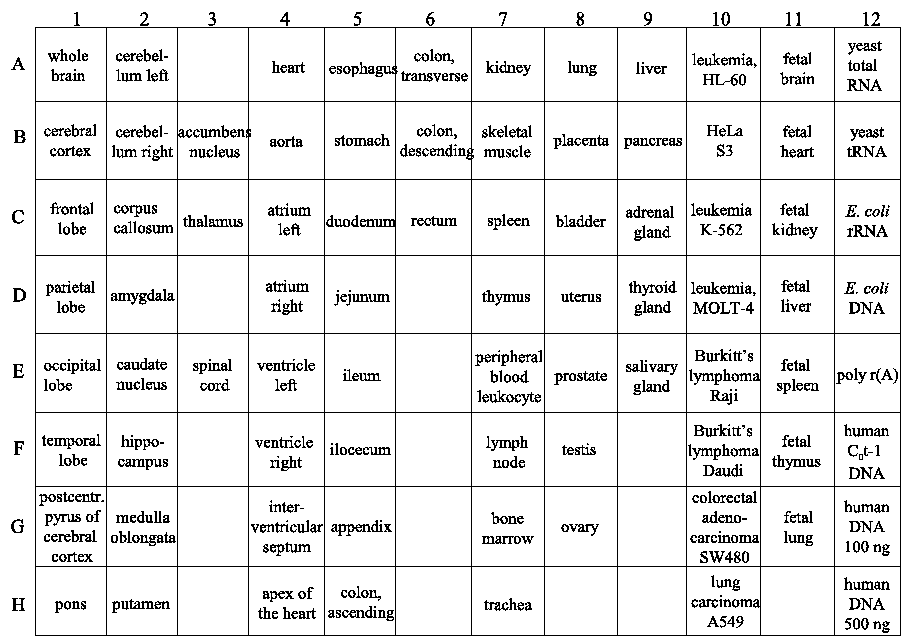
Figure 2 : Transcription profile of the mk5 gene in normal and tumorigenic tissue samples
An MK5-specific probe was used in the hybridization and the results are shown in the top panel. The bottom part of the figure shows a schematic presentation of the type of tissues located on the blot.
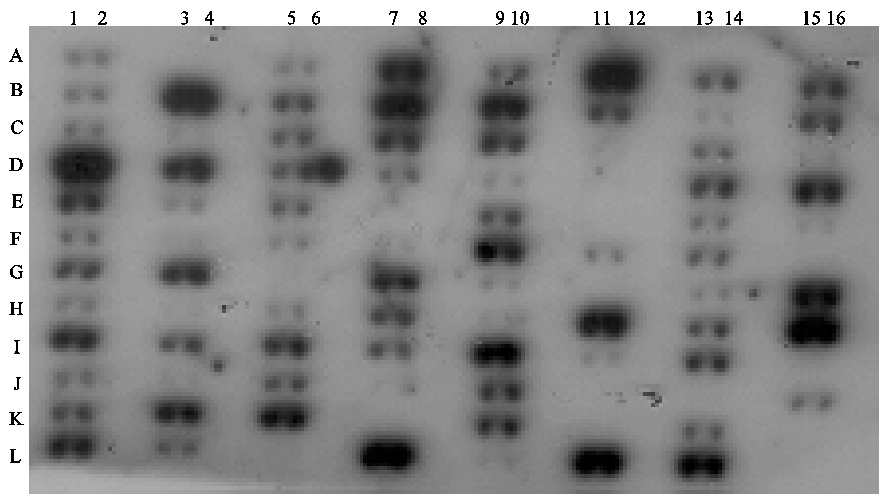
Figure 3 : Putative binding sites for transcription factors in the mouse, rat, and human MK5 promoter
Figure 3A : A region of approximately 3000 bp upstream of the start codon of the mk5 gene was screened for putative binding sites for transcription factors using a transcription factor prediction program. Transcription factor motifs that showed 95% or more identity to known binding consensus sequences are indicated. The sequence of the MK5 promoters is based on available sequences in the GenBank (Benson et al., 2007). A thick red line indicates regions in mouse and rat promoter that have not been sequenced or are ambiguous. This ambiguity is probably the result of the very high GC contents of the promoters.
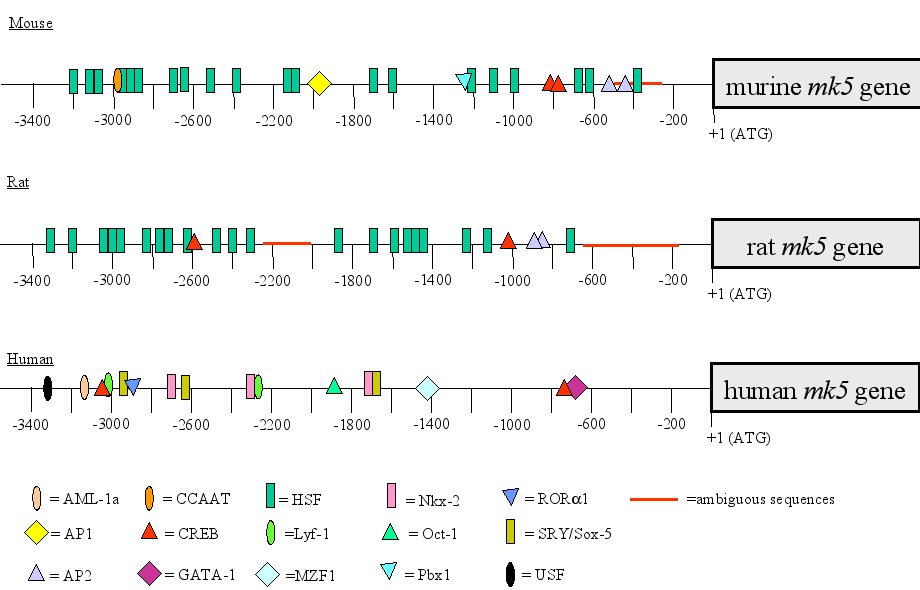
Figure 3B : The sequences and the locations in relation to the start codon of conserved CRE motifs in the MK5 promoter of the three species are shown :
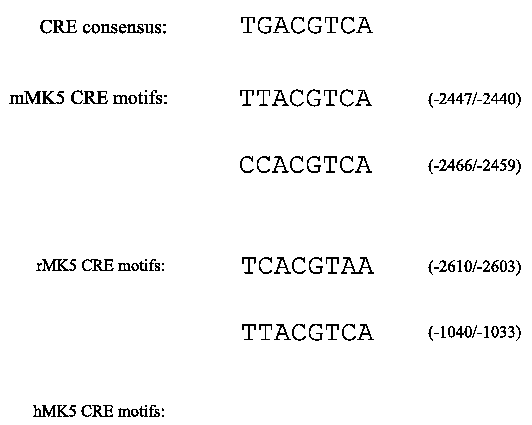
Figure 4 : CREB binds in vitro to the consensus CRE motif in the human MK5 promoter.
Purified CREB protein was incubated with radioactive labeled oligonucleotides with the MK5 CRE motif in the presence or absence of competitor DNA, and DNA-protein complexes were analyzed on an acryl amide gel.
Figure 4A:
Lane 1:
free probe, lanes 2-5:
probe plus recombinant CREB protein. In lanes
3-5 100-fold molar excess of cold competitor
oligonucleotides was added. Lane 3: oligonucleotide with NF·B
binding motif; lane 4:
oligonucleotide with AP1 motif; lane 5:
oligonucleotide with STAT3 motif. Lane 6:
c-fos CRE plus recombinant CREB.
Figure
4B:
Lane 1: free probe, lanes 2-4: MK5 CRE plus
CREB; lane 3: as lane 2, but 100 molar excess of
oligonucleotide with mutated CRE as competitor; lane 4: as
lane 2, but 100 molar excess of oligonucleotide with consensus CRE as
competitor. Lane 5: c-fos CRE plus recombinant CREB.
Figure 5 : Stimulation of the PKA signaling pathway by forskolin induces a transient increase in MK5 transcript levels
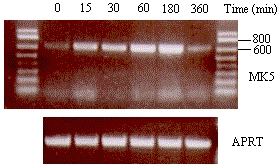 A.
A. 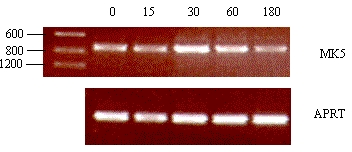 B.
B.
SK-N-DZ (A) or PC12 (B) cells were exposed to 10 ¹M forskolin and mRNA was isolated at different time points after treatment. The mRNA was reversed transcribed and MK5 or APRT transcripts were amplified by PCR with specific primers. The PCR products were visualized by electrophoresis on an agarose gel followed by ethidium bromide staining. A size marker was run on the gel to confirm the correct size of the expected PCR fragment. The time of forskolin stimulation is shown in minutes.
Figure 6 : Forskolin-induced increase in MK5 mRNA results from increased transcription and not stabilization of the transcripts
SK-N-DZ cells were
either pretreated with 10¹M
actinomycin D for 30 min or left untreated before 10¹M
forskolin was added. RNA was subsequently isolated at the time points
indicated and reversed transcribed into cDNA. The presence of MK5
transcripts was assayed by PCR with MK5 specific primers. The
transcript levels of MK5 were compared with the APRT levels. PCR
products were run on an agarose gel and visualized by ethidium
bromide staining.
Figure 7 : The canonical proximal CRE motif of the hMK5 promoter is a poor mediator of cAMP/PKA-induced transcription
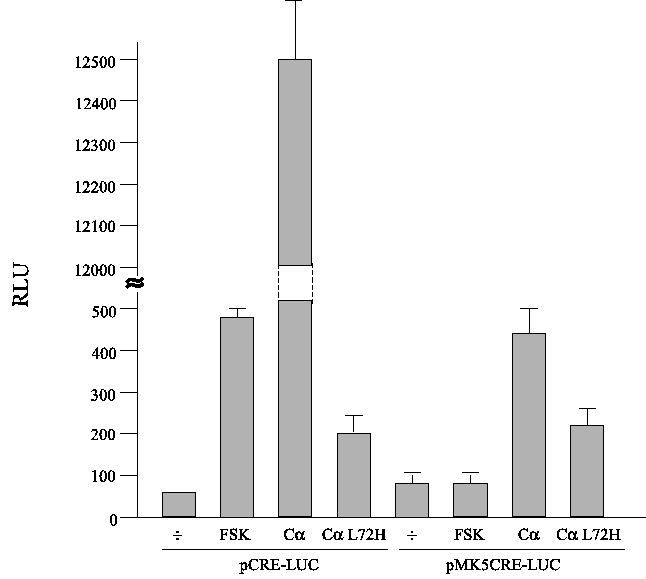
Cells were transiently transfected with a luciferase reporter plasmid and treated with 10 ¹M forskolin for 3 h, or they were cotransfected with an expressing plasmid encoding the catalytic C® subunit of PKA (C®) or a kinase death mutant (C® L72H). The luciferase reporter plasmid contained three copies of the consensus CRE motifs fused to a minimal promoter (pCRE-LUC) or three copies of the proximal hMK5 CRE motif (pCREMK5-LUC). The results show the average (+/-SD) of three independent parallels.
Figure 8 : The VP16-CREB fusion protein moderately stimulates transcription from a minimal promoter containing three copies of the MK5 CRE motif
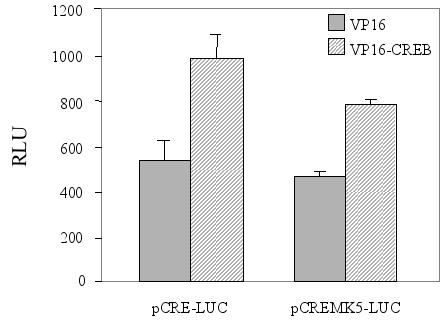
Cells were co-transfected with a luciferase plasmid containing three copies of the consensus CRE motif (pCRE-LUC) or the corresponding plasmid with mutated CRE (pmutCRE-LUC), and an expression vector for either the transactivator domain of VP16 or a fusion protein contain this VP16 moiety and the DNA-binding domain of CREB (VP16-CREB). The results show the average (+/-SD) of three independent parallels.
Figure 9 : The consensus CRE motif in the human MK5 promoter is required for basal promoter activity, but does not mediate cAMP responsiveness
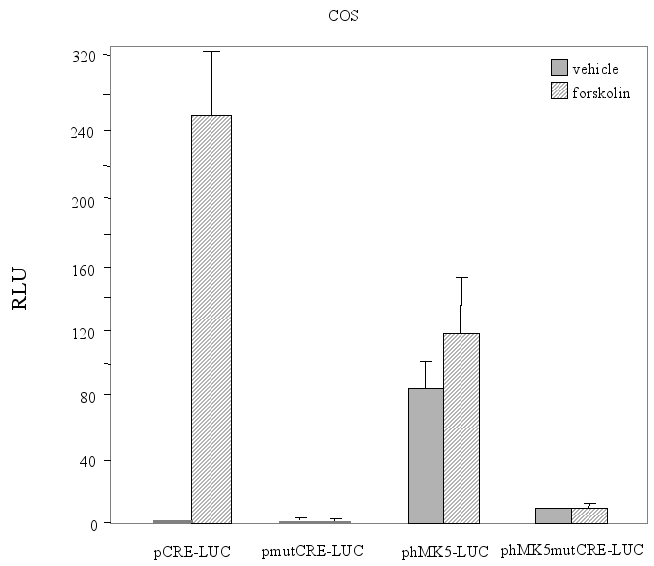
Figure 9A : COS-1 cells were transiently transfected with either a luciferase plasmid containing three copies of the consensus CRE motif (pCRE-LUC) or the corresponding plasmid with mutated CRE (pmutCRE-LUC), or with a reporter plasmid containing an 850 bp fragment of the hMK5 promoter with consensus CRE (phMK5-LUC) or mutated CRE (phMK5mutCRE-LUC). Cells were either treated with vehicle or 10 ¹M forskolin for 3 h and luciferase activity was determined. The results show the average (+/-SD) of three independent parallels. Similar results were obtained in independent experiments.
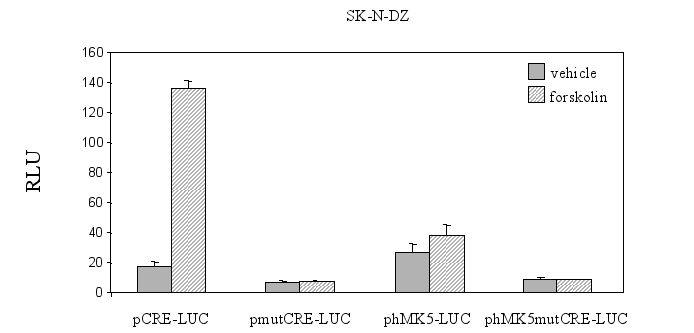
Figure 9B : SK-N-DZ cells were the reporter plasmid containing a 850bp fragment of the hMK5 promoter with consensus CRE (phMK5-LUC) or mutated CRE (phMK5mutCRE-LUC), with control cells transfected with pCRE-Luc and pMutCRE-Luc. Luciferase activity was monitored 24 h after transfection and the results show the average of three independent experiments. The luciferase activity measured in the extracts of cells transfected with the phMK5-LUC construct in the presence of empty vector was arbitrary set as 1.0 and the activities in the other cell extracts is shown as fold-induction.
The same setup was used for PC12 cells (Figure 9C) that were co-transfected with a vector encoding NLS-tagged catalytic subunit (C®) of PKA or empty vector (pRcCMV) and phMK5-LUC or phMK5mutCRE-LUC. Similar results were obtained for both SK-N-DZ and PC12 cells in independent experiments :
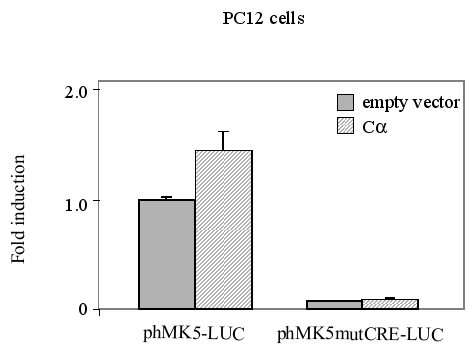
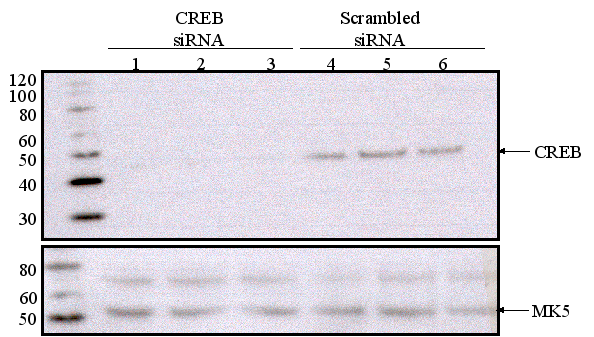
Figure 9D : Depletion of CREB does not affect MK5 expression levels. Three parallel wells of a six-well plate with PC12 cells were transfected with either scrambled siRNA or CREB-targeting siRNA. The protein levels of CREB and MK5 were monitored by western blot 48 h after transfection. The molecular mass (in kDa) of the protein marker is shown.
Figure 10 : Stimulation of the PKA signaling pathway by forskolin does not induce MK5 protein levels
PC12 cells were exposed to 10¹M forskolin for the time points indicated. The amount of MK5 protein was visualized by western blot. To assure equal sample loading, the blot was stripped and re-probed with antibodies against CREB and ERK2. The molecular mass (in kDa) of the protein marker (lane M) is shown.
Figure 11 : The levels of MK5 protein are not affected by several stimuli tested
PC12 cells were serum-starved for 16 h and then treated with different stimuli for the time indicated. Cell extracts were prepared and the amount of MK5 protein was determined by western blot. Membranes were stripped and re-probed with antibodies against CREB to ensure equal loading of the gels. The position of MK5 (~55 kDa) and CREB (~43 kDa) is indicated by an arrow. The molecular mass of the protein marker (M) is indicated.
Figure 11A : PC12 cells were treated with 60 mM KCl or 10 ng/ml(16 nM) TPA:
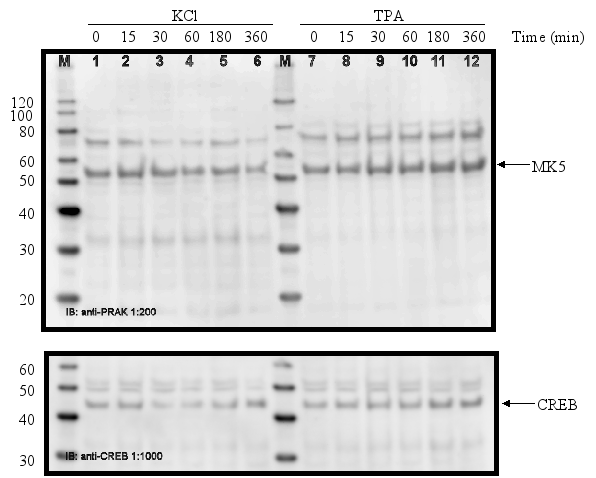
Figure 11B :
Serum-starved cells were treated with 10% foetal bovine serum + 5%
horse serum or with 75 ![]() M
vanadate:
M
vanadate:
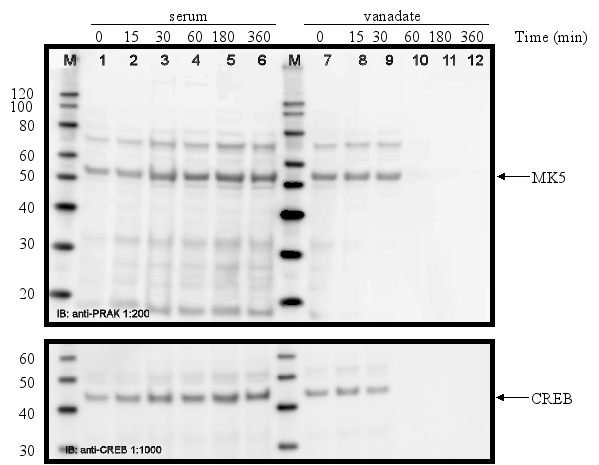
Figure 11C : Cell treated with 250 ¹M arsenite or 500 ¹M spermineNONO:
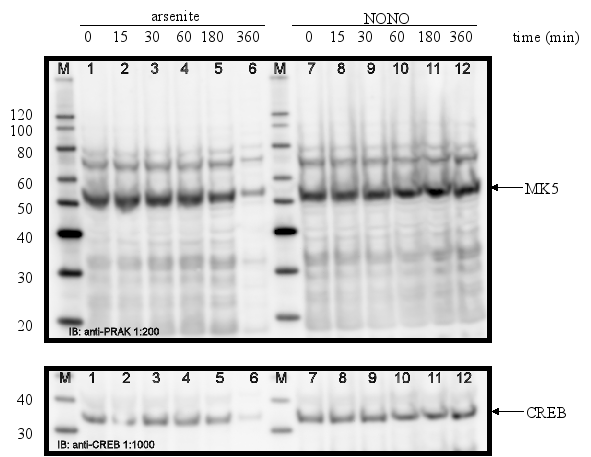
Figure 11D :
Serum-starved cells treated with 500 ng/ml lipopolysaccharides (LPS)
:
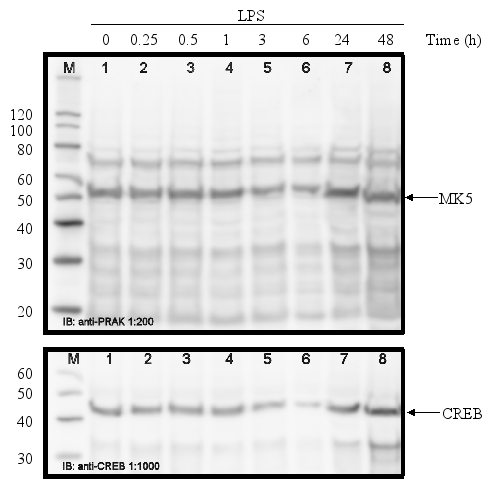
Figure 11E : Serum-starved PC12 cells exposed to 10 ¹M retinoic acid (RA).
Figure 12 : Temperature shock, but not KCl or TPA, induces MK5 transcript levels
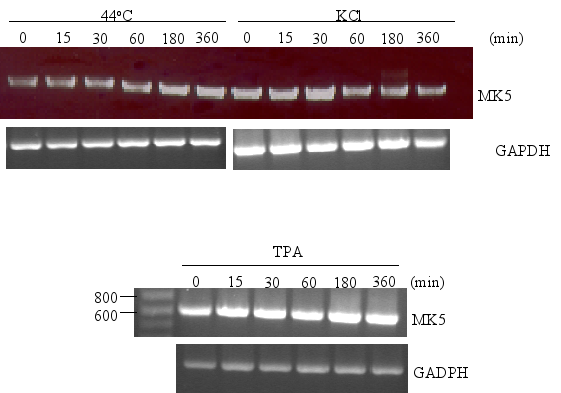
PC12
cells were either exposed to 44°C for 20 min before they were
replaced to 37°C or treated with KCl (60 mM) or TPA (16 nM).
Poly(A)+ RNA was isolated at the time points (in min) indicated and
equal amounts of mRNA were converted into cDNA by reverse
transcriptase. MK5 and GADPH transcripts were amplified by PCR using
specific primers. The PCR products were analyzed by agarose gel
electrophoresis and the products were visualized by ethidium bromide
staining.
Table 1 : Distribution of MK5 mRNA in different adult tissues
Slot blot hybridization was used for the multiple tissue arrays of Clontech and BioChain, while northern blotting was applied in the works of Ni et al. and New et al. The absence of MK5 mRNA is indicated by "-", while the presence is shown as "+". * Designates that several samples of the same tissue were placed on different areas of the multiple tissue array. Open field in the columns indicates that the tissue was not tested.
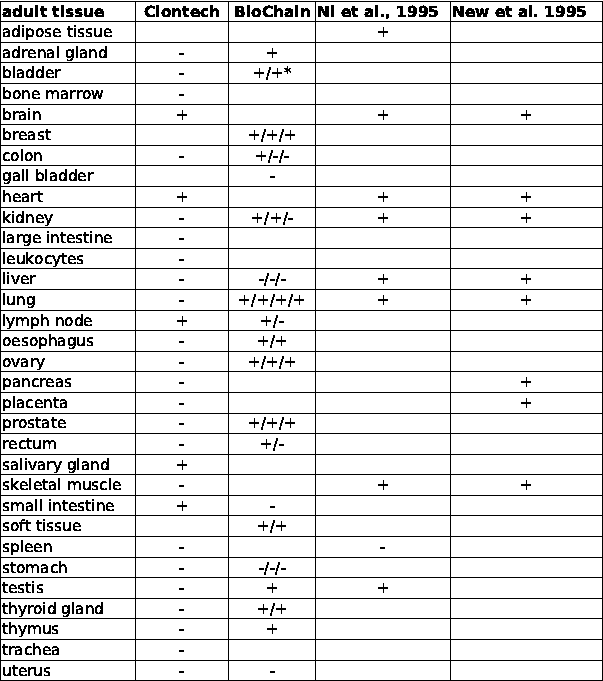
Table 2 : Function, expression pattern, and binding sequence of the transcription factors with putative binding site in the mouse, rat,and human MK5 promoter
R= A or G and Y= C or T
|
transcription factor |
consensus binding motif |
function |
expression pattern |
reference |
|
AML-1a (=CBF2) |
AACCACA |
regulation of transcription of haematopoietic and bone-specific genes; early haematopoietic development |
haematopoietic cells, thymus |
Choi et al., 2003 |
|
AP1 |
TGACTCA |
transcription of gene regulation networks involved in cell proliferation and apoptosis |
ubiquitously |
Eferl and Wagner, 2003 |
|
AP2 |
GCC(N)3GGC |
cell-type-specific stimulation of proliferation and suppression of terminal differentiation during embryonic development |
ubiquitously |
Eckert et al., 2005 |
|
CCAAT/enhancer binding protein (C/EBP) |
RTTGCGYAAY |
tumour suppressor, development and in tissue homeostasis, inflammatory response |
tissue-specific manner |
Ramji and Foka, 2002 |
|
CREB |
TGACGTCA |
transcription of gene regulation networks involved in metabolism, memory and learning processes, immunity |
ubiquitously |
Johannessen and Moens, 2005 |
|
GATA-1 |
A/TGATAA/G |
transcription of gene regulation networks involved in haematopoiesis |
erythroid, megakaryocytic, eosinophil and mast cell lineages |
Lowry and Mackay, 2006 |
|
HSF |
NGAAN |
transcription of gene regulation networks involved in protein folding, processing, targeting, degradation, and function, stress response |
ubiquitously |
Morano and Thiele, 1999 |
|
Lyf-1 (=Ikaros) |
CCTCCCAAA |
transcription of gene regulation networks involved in haematopoiesis |
haematopoietic cells |
Lo et al., 1991; Han et al., 2007 |
|
MZF1 |
GTGGGG |
differentiation program myeloid progenitor cells |
myeloid progenitor cell and totipotent haemopoietic cells |
Yan et al., 2006 |
|
Nkx-2 |
TNAAGTG |
organ specific development, especially brain and heart |
neural progenitor cells; heart, spleen, stomach |
McMahon, 2000 |
|
Oct-1 |
ATGCAAATC |
Transcriptional regulation, DNA replication, apoptosis, transcription of gene regulation networks involved in stress |
ubiquitously |
Tantin et al., 2005 |
|
Pbx1 |
ATCAATCAA |
developmental gene regulation |
ubiquitously, except B- and T-cells |
Van Dijk et al., 1995 |
|
ROR1 |
ATAACTAGGTCA |
lipid metabolism, immune function, and bone development. |
ubiquitously |
Gold et al., 2007 |
|
Sox-5/SRY |
AACAAT |
regulation cell fate during spermatogenesis |
testis |
Denny et al., 1992 |
|
USF |
CACGTG |
transcription of gene regulation networks, including the stress and immune responses, cell cycle and proliferation, lipid and glucid metabolism, |
ubiquitously |
Corre and Galibert, 2005 |
Bibliography
- 1
-
P. Coulombe, G. Rodier, S. Pelletier, J. Pellerin,
and S. Meloche.
Rapid turnover of extracellular signal-regulated kinase 3 by the ubiquitin-proteasome pathway defines a novel paradigm of the mitogen-activated protein kinase regulation during cellular differentiation.
Molecular and Cellular Biology, 23(13):4542-4558, 2003. - 2
-
D. Crowe, R. Kim, and R. Chandraratna.
Retinoic acid differentially regulates cancer cell proliferation via dose-dependent modulation of the mitogen-activated protein kinase pathway.
Molecular Cancer Research, 1:532-540, 2003. - 3
-
N. DeRuiter, R. Wolthuis, H. vanDam, B. Burgering,
and J. Bos.
Ras-dependent regulation of c-Jun phosphorylation is mediated by the Ral guanine nucleotide exchange factor-Ral pathway.
Molecular and Cellular Biology, 20:8480-8488, 2000. - 4
-
D. Fambrough, K. McClure, A. Kazlauskas, and
E. Lander.
Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes.
Cell, 97:727-741, 1999. - 5
- D. Fass,
J. Butler, and R. Goodman.
Deacetylase activity is required for cAMP activation of a subset of CREB target genes.
The Journal of Biological Chemistry, 278:43014-43019, 2003. - 6
- S. Fu,
W. Chou, D. Huang, and H. Huang.
Transcriptional regulation of a rice mitogen-activated protein kinase gene, OsMAPK4, in response to environmental stresses.
Plant Cell Physiology, 43:958-963, 2002. - 7
-
D. Fusco, N. Accornero, B. Lavole, S. Shenoy,
J. Blanchard, R. Singer, and E. Bertrand.
Single mRNA molecules demonstrate probabilistic movement in living mammalian cells.
Current Biology, 13:161-1677, 2003. - 8
- W. Hong,
C. He, L. Wang, D. Wang, L. Joseph,
C. Jantasuriyarat, L. Dai, and G. Wang.
BWMK1 responds to multiple environmental stresses and plant hormones.
Journal of Integrative Plant Biology, 49:843-851, 2007. - 9
-
H. Huang, S. Fu, Y. Tai, W. Choi, and
D. Huang.
Expression of Oryza sativa MAP kinase gene is developmentally regulated and stress-responsive.
Physiologica Plantarum, 114:572-580, 2002. - 10
-
M. Imagawa, R. Chiu, and M. Karin.
Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP.
Cell, 51:251-260, 1987. - 11
-
S. Imagawa, S. Fujii, J. Dong, T. Furumoto,
T. Kaneko, T. Z. Y. Satoh, Tsutsui, and B. Sobel.
Hepatocyte growth factor regulated E box-dependent plasminogen activator inhibitor type 1 gene expression in HepG2 liver cells.
Arteriosclerosis, Thrombosis and Vascular Biology, 26:2407-2413, 2006. - 12
-
M. Imajo, Y. Tsuchiya, and E. Nishida.
Regulatory mechanisms and functions of MAP kinase signaling pathways.
IUBMB Life, 58(5-6):312-317, 2006. - 13
-
S. Impey, S. R. McCorkle, H. Cha-Molstad, J. M.
Dwyer, G. S. Yochum, J. M. Boss, S. McWeeney, J. J.
Dunn, G. Mandel, and R. H. Goodman.
Defining the CREB regulon: a genome-wide analysis of transcription factory regulatory regions.
Cell, 119:1041-1054, 2004. - 14
-
M. Johannessen, M. P. Delghandi, O. M. Seternes,
B. Johansen, and U. Moens.
Synergistic activation of CREB-mediated transcription by forskolin and phorbol ester requires PKC and depends on the glutamine-rich Q2 transactivation domain.
Cellular Signalling, 16:1187-1199, 2004. - 15
-
M. Johannessen and U. Moens.
Trends in Cellular Signalling, chapter Transcription of genes in response to activated cAMP/protein kinase A signalling pathway : There is more to it than CREB.
Nova Publisher, 2005. - 16
-
M. Johannessen and U. Moens.
Multisite phosphorylation of the cAMP response element-binding protein (CREB) by a diversity of protein kinases.
Frontiers in Biosciences, 12:1814-1832, 2007. - 17
- S. Kim,
J. Hong, S. Ryu, W. Chung, J. Yoon, and
J. Koo.
Regulation of mucin gene expression by CREB via a nonclassical retinoic acid signalling pathway.
Molecular and Cellular Biology, 27:6933-6947, 2007. - 18
-
K. Kitta, R. Day, Y. Kim, I. Torregroza,
T. Evans, and Y. Suzuki.
Hepatocyte growth factor induces GATA-4 phosphorylation and cell survival in cardiac muscle cells.
The Journal of Biological Chemistry, 278:4705-4712, 2002. - 19
- W. Lee,
P. Mitchell, and R. Tjian.
Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements.
Cell, 49:741-752, 1987. - 20
-
B. Luscher, P. Mitchell, T. Williams, and R. Tjian.
Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers.
Genes and Development, 3:1507-1517, 1989. - 21
- S. Ma
and H. Bohnert.
Integration of Arabidopsis thaliana stress-related transcript profiles, promoter structures, and cell-specific expression.
Genome Biology, 8:R49, 2007. - 22
- E. T.
Maizels, A. Mukherjee, G. Sithanandam, C. A. Peters,
J. Cottom, K. E. Mayo, and M. Hunzicker-Dunn.
Developmental regulation of mitogen-activated protein kinase-activated kinases-2 and -3 (MAPKAPK-2/-3) in vivo during corpus luteum formation in the rat.
Molecular Endocrinology, 15(5):716-733, 2001. - 23
-
G. Manning, D. Whyte, R. Martinez, T. Hunter, and
S. Sudarsanam.
The protein kinase complement of the human genome.
Science, 298:1912-1934, 2002. - 24
-
T. Mikalsen, M. Johannessen, and U. Moens.
Sequence- and position-dependent tagging protects extracellular-regulated kinase 3 protein from 26S proteasome-mediated degradation.
International Journal of Biochemistry and Cell Biology, 37:2513-2520, 2005. - 25
-
Y. Mizoguchi, K. Irie, T. Hirayama, N. Hayashida,
K. Yamaguchi-Shinozaki, K. Matsumoto, and K. Shinozaki.
A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana.
Proceedings of the National Academy of Sciences of the USA, 93:765-769, 1996. - 26
-
U. Moens, N. Subramaniam, B. Johansen, T. Johansen,
and T. Traavik.
A steroid hormone response unit in the late leader of the noncoding control region of the human polyomavirus bk confers enhanced host cell permissivity.
Journal of Virology, 68:2398-2408, 1994. - 27
-
M. Montminy.
Transcriptional regulation by cyclic AMP.
Annual Reviews in Biochemistry, 66:807-822, 1997. - 28
-
D. Natale, A. Paliga, F. Beier, S. D'Souza, and
A. Watson.
p38 MAPK signaling during murine preimplantation development.
Developmental Biology, 268(1):76-88, 2004. - 29
- L. New,
Y. Jiang, M. Zhao, K. Liu, W. Zhu, L. J.
Flood, Y. Kato, G. C. Parry, and J. Han.
PRAK, a novel protein kinase regulated by p38 MAPK.
The EMBO Journal, 17(12):3372-3384, 1998. - 30
- H. Ni,
X. Wang, K. Diener, and Z. Yao.
MAPKAPK5, a novel MAPK activated protein kinase is substrate of the extracellular regulated kinase (ERK) and p38 kinase.
Biochemical and Biophysical Research Communications, 243:492-496, 1998. - 31
- A. J.
Paliga, D. R. Natale, and A. J. Watson.
p38 mitogen-activated protein kinase (MAPK) first regulates filamentous actin at the 8-16-cell stage during preimplantation development.
Biological Cell, 97:629-640, 2005. - 32
-
M. Raman, W. Chen, and M. Cobb.
Differential regulation and properties of mapks.
Oncogene, 26:3100-3112, 2007. - 33
-
M. Rasar, D. DeFranco, and S. R. Hammes.
Paxillin regulates steroid-triggered meiotic resumption in oocytes by enhancing an all-or-none positive feedback kinase loop.
The Journal of Biological Chemistry, 281:39455-39464, 2006. - 34
-
K. Ravnskjaer, H. Kester, Y. Liu, X. Zhang,
D. Lee, J. R. YatesIII, and M. Montminy.
Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression.
EMBO Journal, pages 1-10, 2007. - 35
-
L. Reynolds and R. Richards.
Can toxicogenomics provide information on the bioreactivity of diesel exhaust particles?
Toxicology, 165:145-152, 2001. - 36
-
R. Ryseck and R. Bravo.
c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins.
Oncogene, 6:533-542, 1991. - 37
- P. J.
Santangelo, B. Nix, A. Tsourkas, and G. Bao.
Dual FRET molecular beacons for mRNA detection in living cells.
Nucleic Acids Research, 32(6):e57, 2004. - 38
-
E. Schneider, M. Weiss, W. du, G. Leder,
K. Buttenschön, U. Liener, and U. Brückner.
MAPkinase gene expression, as determined by microarray analysis, distinguishes uncomplicated from complicated reconstitution after major surgical trauma.
Annals of the New York Academy of Sciences, 1090:429-444, 2006. - 39
- O. M.
Seternes, T. Mikalsen, B. Johansen, E. Michaelsen,
C. G. Armstrong, N. A. Morrice, B. Turgeon,
S. Meloche, U. Moens, and S. M. Keyse.
Activation of MK5/PRAK by the atypcial MAP kinase ERK3 defines a novel signal transduction pathway.
The EMBO Journal, 23:4780-4791, 2004. - 40
- F. Song
and R. Goodman.
OsBIMK1, a rice MAP kinase gene involved in disease resistance responses.
Planta, 215:997-1005, 2002. - 41
-
Z. Travnickova-Bendova, N. Cermakian, S. M. Reppert, and
P. Sassone-Corsi.
Bimodal regulation of mPeriod promotors by CREB-dependent signaling and CLOCK/BMAL1 activity.
Proceedings of the National Academy of Sciences, 99(11):7728-7733, 2002. - 42
- L. J.
Vician, G. Xu, W. Liu, J. D. Feldman, H. B.
Machado, and H. R. Herschman.
MAPKAP kinase-2 is a primary response gene induced by depolarization in PC12 cells in brain.
Journal of Neuroscience Research, 78:315-328, 2004. - 43
- N. Vo,
M. Klein, O.Varlamova, D. Keller, T. Yamamoto,
R. Goodman, and S. Impey.
A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis.
Proceedings of the National Academy of Sciences of the USA, 102:16426-16431, 2005. - 44
-
E. Wingender, X. Chen, R. Hehl, H. Karas,
I. Liebich, T. Matys, T. Meinhardt, M. Pruss,
I. Reuter, and F. Schacherer.
Transfac: an integrated system for gene expression regulation.
Nucleic Acids Research, 28:316-319, 2000. - 45
-
X. Zhang, D. T. Odom, S.-H. Koo, M. D. Conkright,
C. Canettieri, J. Best, H. Chen, R. Jenner,
E. Herbolsheimer, E. Jacobsen, S. Kadam, J. R.
Ecker, B. Emerson, J. B. Hogenesch, T. Unterman,
R. A. Young, and M. Montminy.
Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation and target gene activation in human tissues.
Proceedings of the National Academy of Science, 102:4459-4464, 2005.
About this document ...
This document was generated using the LaTeX2HTML translator Version 2002-2-1 (1.71)
Copyright © 1993, 1994, 1995, 1996, Nikos
Drakos, Computer Based Learning Unit, University of Leeds.
Copyright
© 1997, 1998, 1999, Ross
Moore, Mathematics Department, Macquarie University, Sydney.